Atrial Fibrillation (AF)
AF is suspected from symptoms of ineffective cardiac output (syncope or cognitive changes), palpitations, tachycardia, fatigue, or dyspnea; or signs that include an irregularly irregular pulse.
What is Atrial Fibrillation (AF)?
Atrial premature beats are an important trigger for atrial fibrillation. In on-going AF, there is no organized wave of depolarization that sets up an organized contraction. The atrium basically quivers without an organized net vector force to drive blood into the ventricles normally for effective cardiac output.
The ventricles and their own pacemaker, the atrioventricular node, can “rescue” the process with their own automaticity, but this is not as effective a pump.
AF is usually due to some underlying heart disease, atrial enlargement, irritation, or inflammation:
Hypertension.
Coronary heart disease (CHD).
Previous myocardial infarction.
Rheumatic heart disease (RHD) and other valvular abnormalities.
Heart failure.
Cardiomyopathy–disease of the heart muscle, associated with hypertrophy.
Congenital heart disease (CHD).
Chronic obstructive pulmonary disease (COPD).
Obstructive sleep apnea.
Obesity.
Diabetes.
Hyperthyroidism.
Heart surgery.
Medications (theophylline, adenosine, and drugs for osteoporosis).
AF is classified as new-onset, paroxysmal, persistent, long standing persistent, or permanent.
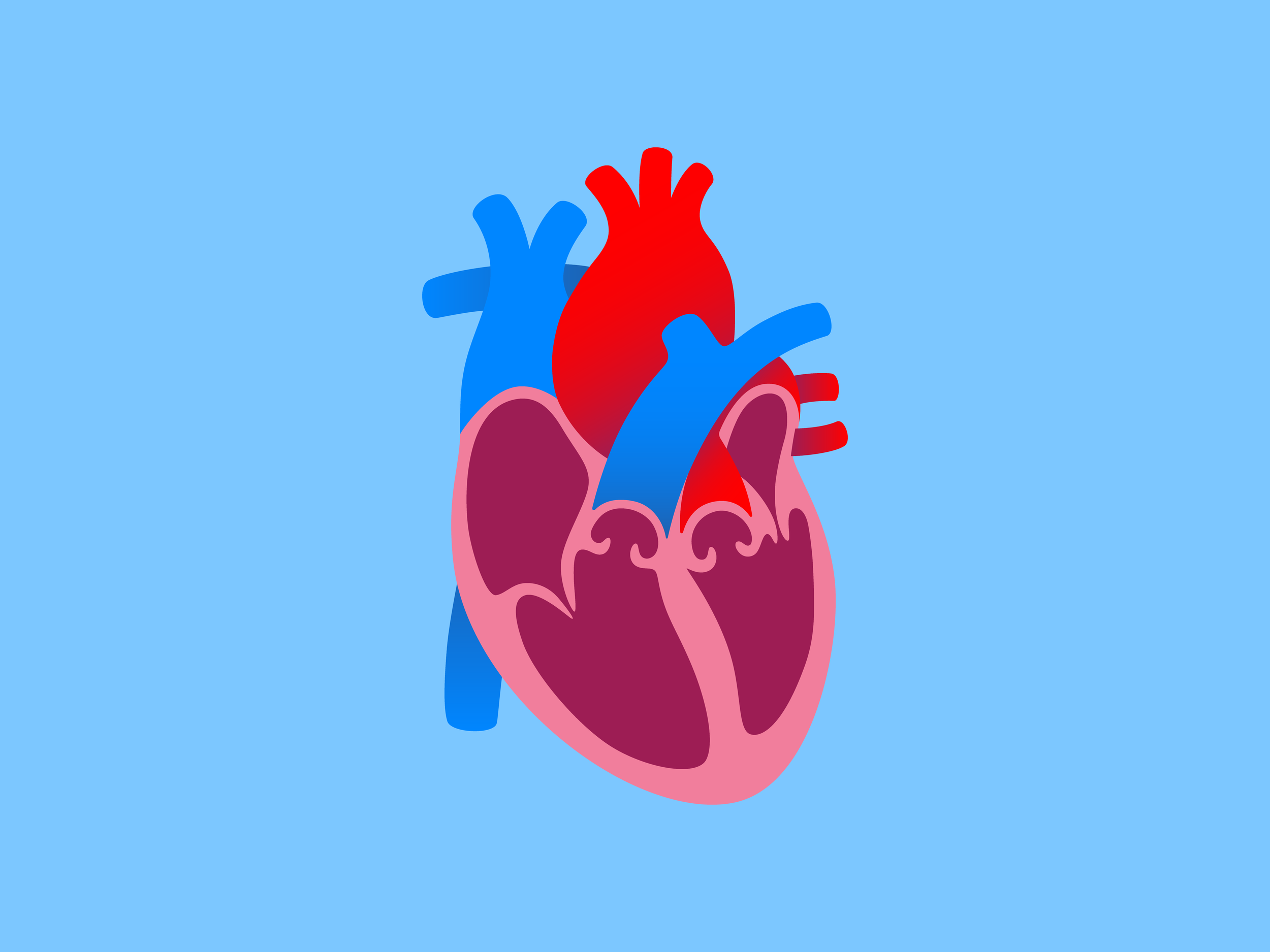
The Atria
The right and left atria (singular, atrium) receive blood from the vena cava (inferior and superior) and the pulmonary veins, respectively. This dichotomy represents a division between deoxygenated blood and oxygenated blood, with the lungs between these two circuits. From the atria, which contract, blood is sent to the right and left ventricles, which then send it to the lungs or to the rest of the body, respectively. Thus, the heart is a 4-pump mechanism, i.e., the two atria and the two ventricles.
The cells of the heart have automaticity, sending electrical pulses in sequence. The sinoatrial (SA) node is the “pacemaker” that supersedes all of the other cells’ automaticity and determines the rhythm of contractions in the heart, which generally travel right to left and superior to inferior. These contractions are skewed in their timing such that there is one vector force that propels the blood onward. One-way valves prevent backflow.
Other Atrial Arrhythmias
Supraventricular arrhythmia, as opposed to rogue automaticity from disease in the sinoatrial node or ectopic sites of automaticity, involves re-entry of electrical signals into the circuit.
Increased vagal tone, low serum magnesium (hypomagnesemia), alcohol, and caffeine have all been known to be triggers for atrial fibrillation (AF).
Atrial flutter differs from AF by having a regular rhythm and identifiable P waves. Its rate can be 250-350 beats/minute.
Complications from AF
AF impairs cardiac output, leading to symptoms of syncope, near-syncope, and other cognitive impairment. It is also a prime cause of arterial emboli that can cause stroke and peripheral embolization, requiring anticoagulant therapy.
How is Atrial Fibrillation (AF) diagnosed?
AF is suspected from symptoms of ineffective cardiac output (syncope or cognitive changes), palpitations, tachycardia, fatigue, or dyspnea; or signs that include an irregularly irregular pulse. It is often associated with exercise, emotional stress, or alcohol.
A medical history for conditions associated with AF, such as diabetes or stroke, can lead to a diagnostic workup that includes electrocardiogram (ECG) or long-term monitoring, such as with a Holter monitor. Occasionally, such continuous monitoring done for other reasons may pick up an AF arrhythmia incidentally.
Anyone who has previously had or is currently experiencing a cerebrovascular accident (stroke) or other arterial thromboembolism, for which AF is a major cause, should be evaluated for AF.
Physical exam may demonstrate an irregularly irregular pulse. (Some irregular pulses have patterns, but not with AF).
Electrocardiogram (ECG)
An ECG can discern abnormalities in the electrical conduction through the heart. Each chamber of the heart has a characteristic depolarization wave that is seen on ECG with a contraction, but in atrial fibrillation, its wave–the P wave–is absent, the atrial quivering consistent with a rate of firing of >300/minutes. There is no consistent beat-to-beat rhythm nor any repetitive patterns.
Echocardiography
An ultrasound of the heart is used to identify the structure and function of the atria and ventricles and to identify any valve abnormalities. Thrombi in the atrium can be identified to further justify the rationale for anticoagulation therapy.
Stress Testing
Stress testing in conjunction with ECG and echocardiography can be used to determine heart rate control in those prone to AF.
Blood Tests
Tests for other causes, such as thyroid function testing, serum creatinine for kidney appraisal, and blood sugar to screen for diabetes are included in the diagnostics for AF.
How can I manage Atrial Fibrillation (AF)?
With the morbidity and mortality associated with AF, it is prudent to identify it quickly and treat it to prevent life-threatening impairment of cardiac output, stroke, cognitive changes, and cardiac death. Those with additional cardiac disease, e.g., coronary artery disease, heart failure, and hypertension, have augmented risks. In any management of AF, reversible triggers should be eliminated, such as hyperthyroidism and hyperglycemia. If a patient is hemodynamically unstable, management by an intensivist in a cardiac care facility is necessary, including immediate cardioversion and anticoagulation. A patient is considered unstable if there is sever bradycardia (slow rate) or prolonged pauses or intervals during each cycle. For those who are hemodynamically stable, restoring a normal sinus rhythm is done via a more methodical course of cardioversion.
Cardioversion
Cardioversion treatment involves restoring a normal sinus rhythm. There are two ways to accomplish cardioversion:
Oral beta blockers or angiotensin-converting-enzyme (ACE) inhibitors.
Electrical (DC shock).
The electrical cardioversion has a better success rate than the chemical approach, but the decision of which to use is individualized based on patient condition, comorbidities, age, patient preference after informed consent, and physician judgment. One factor that is considered is that antiarrhythmic drugs themselves can cause arrhythmias. Alternately, oral medication does not involve sedation and anesthesia which may be contraindicated in some individuals. Antiarrhythmic drugs are used after cardioversion to maintain the gains that electrical stimulation creates by reestablishing a normal sinus rhythm.
New-onset AF
A person with his or her first episode of AF should undergo cardioversion, since there are some AF patients who will never have a second episode. AF is a condition that affects both cardiac rate and rhythm, and of these, rate control is the first step (unless the patient is unstable). Beta blockers or ACE inhibitors are used until there is a rate of 90 or less. After the rate is controlled, attention to the rhythm is next, via cardioversion.
Longstanding or recurrent AF
There are two goals for patients with AF:
Symptom control.
Prevention of thromboembolism.
Rate control (generally for those >65 years of age) is achieved with blockage of the propagation of the rapid atrial impulses at the next stage of cardiac conduction to the ventricles at the atrioventricular (AV) node. Medications used include rate-slowing calcium-channel blockers, beta blockers, or digoxin. Rhythm control (generally for those <65 years of age) is achieved using antiarrhythmic drugs. This choice is primarily centered on relieving the symptoms that AF produces.
Anticoagulation
With the increased risk of thromboembolism (and its tendencies toward stroke and death), atrial fibrillations association with thrombus formation is countered with an anticoagulation strategy.
How can I prevent Atrial Fibrillation (AF)?
AF may not be able to be prevented if underlying genetics, chronic obstructive pulmonary disease (COPD), or other irreversible triggers contribute to its emergence. However, reversible causes can and should be addressed by treating any hyperthyroidism, smoking cessation, strict glycemic control in diabetics, weight management to address obesity and obstructive sleep apnea, and other indicated interventions into associated underlying causes.
With an established diagnosis of AF, there are two main goals in preventable intervention:
Prevention or mitigation of symptoms.
Prevention of arterial thromboembolism.
Prevention of symptoms
Correction of either the rate or rhythm as a first step or–ideally–establishing a normal sinus rhythm can address the symptoms that come from the impaired cardiac output that atrial fibrillation creates. With underlying coronary vascular disease, the tachycardia may cause angina. Other symptoms such as palpitations, dyspnea on exertion, lightheadedness, fatigue, syncope, or generalized malaise can be lessened or eliminated with reestablishing an efficient pumping action.
Prevention of Embolism
With AF ineffective pump action in the atria, blood is not effectively pumped into the ventricles as well as with normal sinus rhythm, so this change in fluid dynamics makes more likely coagulation effects that result in thrombi. In the left atrium, such thrombi can break off as emboli and enter the systemic circulation via the left ventricle. This can result in emboli being sent to the cerebral circulation (as an obstructive, ischemic stroke) or peripheral thromboembolism, putting target organs in harm’s way (e.g., mesenteric artery resulting in intestinal ischemia and bowel necrosis).
Thrombus formation is prevented by anticoagulation, and various regimens are determined by whether there already has been an embolic event, the absence of thrombi on echocardiogram, or thrombus presence without emboli to date. Essentially, however, almost all AF patients are anticoagulated, as recommended by the National Institutes of Health, unless there is a contraindication.
Anticoagulation is intervening into the clotting cascade to lengthen the timing and briskness of clot formation, and a therapeutic range is kept constant via surveillance with clotting studies. Newer anticoagulants eliminate the need for routine clotting studies, so in patients taking these, the surveillance parameters are adjusted accordingly.